Trona: The Mineral and the Lake That Made It Famous
When people hear the word “Trona,” they might think first of the tiny desert town in California’s Searles Valley. But long before there was a town, there was a mineral—trona itself—and long before that, there was a great Ice Age lake that left behind an astonishing chemical treasure chest. This is the story of the mineral, the lake, and the wider desert world it belongs to.
The Mineral Trona
Trona is a sodium carbonate mineral with the chemical formula Na3(CO3)(HCO3)·2H2O. At first glance, it doesn’t look impressive—grayish, sometimes yellowish, dull and earthy in texture. You can scratch it with a fingernail, since it ranks only 2.5 on the Mohs scale. But appearances deceive. Trona is the raw material that fuels the soda ash industry, and soda ash in turn is essential for making glass, detergents, paper, and countless industrial products.
Trona forms in dry, alkaline lakes where evaporation pulls water away and leaves dissolved salts behind. In crystal form, it can be fibrous, granular, or massive, and it’s often found alongside related salts like nahcolite, gaylussite, and halite. It belongs to the evaporite family—minerals that crystallize in the desert sun as lakes vanish.
Trona’s Desert Cousins
To understand trona, you have to meet its “cousins.” Borax, another evaporite mineral, forms in highly alkaline, boron-rich lakes. Borax is white and powdery, long known for its role in glassmaking and cleaning products, and it gained fame during the “20 Mule Team” borax days of Death Valley. Hanksite, a rare mineral found almost exclusively in Searles Lake, is more exotic. With its strange mix of carbonate, sulfate, and chloride, it grows in large hexagonal crystals prized by collectors. Thenardite, a sodium sulfate, appears later in the drying sequence, often after trona has already crystallized. And halite—plain rock salt—is the final curtain call in the evaporation process, forming thick beds of common salt.
Together, these minerals tell the chemical story of ancient lakes. Each one signals a different stage in the drying cycle: carbonates like trona first, then borates, then sulfates, then chlorides, with rare mixes like hanksite reserved for the most concentrated brines.
The Evaporation Sequence
Picture a great lake in the desert slowly drying under the sun. As water disappears, minerals crystallize out in a sequence. First come the carbonates—trona and nahcolite—as the lake becomes alkaline. If boron is present, borax forms next. With more evaporation, sodium sulfate minerals like thenardite precipitate. Finally, halite—rock salt—deposits in massive beds, and in the last brine stages, unusual combinations like hanksite grow. It’s nature’s chemistry experiment written on the desert floor.
Searles Lake: A Desert Laboratory
Searles Lake in the Mojave Desert is one of the best examples of this process. During the Ice Age, between 30,000 and 12,000 years ago, Searles Lake was a deep freshwater body. Fed by the Mojave River, it was part of a chain of lakes stretching across the desert. When the climate dried, Searles Lake shrank and minerals built up, cycle after cycle, until its bed became one of the richest evaporite deposits on earth.
Today, companies mine Searles Lake for trona, borax, soda ash, sodium sulfate, and halite. They use solution-mining, pumping brines from beneath the playa into evaporation ponds, then refining the salts. The nearby town of Trona, California, owes its name and existence to this mineral wealth. Each October, the community hosts “Gem-O-Rama,” when collectors from around the world flock to dig crystals of hanksite, borax, and halite fresh from the brines.
The Mojave River Lake Chain
Searles Lake didn’t exist in isolation. It was one link in a chain of Ice Age lakes connected by the Mojave River. The river began in the San Bernardino Mountains, flowed through the Victor Valley, and filled Lake Manix near Barstow. When Lake Manix spilled north through Afton Canyon, water reached Soda and Silver Lakes, forming Lake Mojave. In the wettest periods, Lake Mojave overflowed into Searles Lake. From there, water sometimes spilled farther into Panamint Valley, filling Panamint Lake. In the very wettest times, the chain reached all the way to Death Valley, where Lake Manly stretched 80 miles long and hundreds of feet deep.
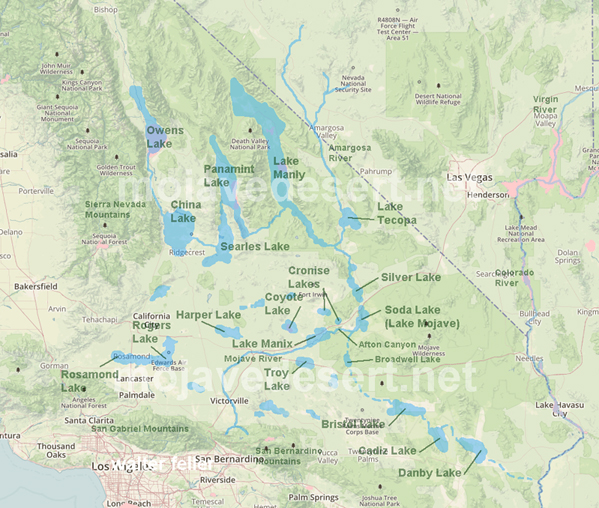
This chain acted like a conveyor belt for water and minerals. Each basin concentrated dissolved salts, but Searles Lake, being closed and rarely flushed, became a perfect trap. That is why its sediments are so rich in trona and other evaporites.
From Ice Age to Industry
When the Ice Age ended, about 12,000 years ago, the climate warmed, and the Mojave River carried far less water. Searles Lake dried into a playa, but its mineral layers remained. By the 1800s, prospectors discovered borax there. In 1873, John and Dennis Searles began mining borax with mule teams, one of the earliest borax operations in California. By the early 1900s, large-scale industrial mining was underway, and the town of Trona was established to house workers. Throughout the 20th century, operations expanded with new methods, and today the lake continues to support a thriving industry and a small desert community.
A Legacy Written in Salt
Searles Lake is more than a dry playa; it is a book of climate history, a record of ancient rivers, and a living source of industrial minerals. The mineral trona, dull to look at but essential to modern life, is both its namesake and its most important product. In the larger story of the desert, Searles Lake shows how geology, chemistry, and human enterprise meet in one stark basin. From Ice Age waters to the shelves of today’s stores, trona’s journey is a desert tale written in crystal layers.
–